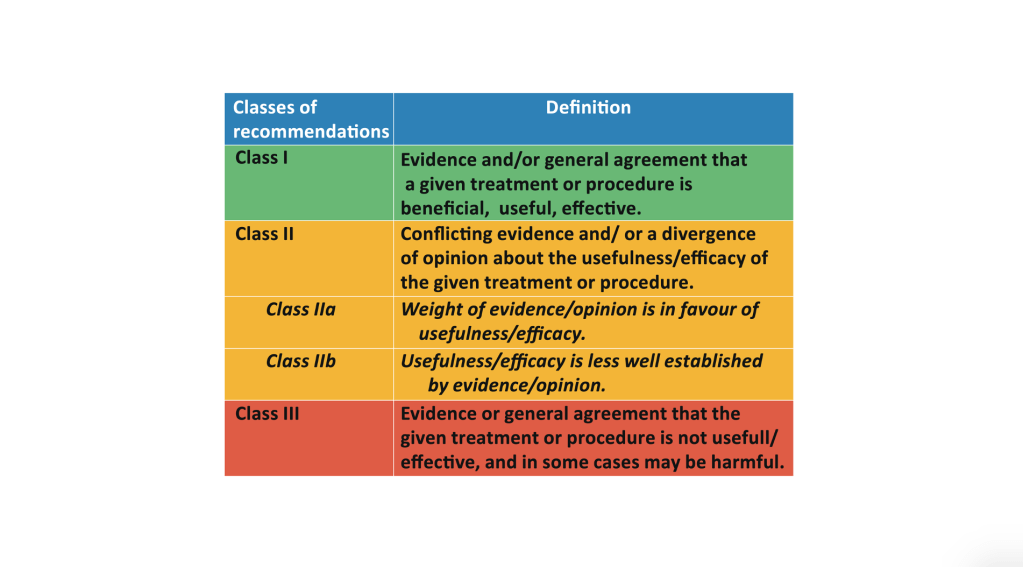
Write your own headline. Choose one from each category below, fiddle with the grammar so it makes sense and bingo!
| NHS organisation | Did something bad | Resulting in |
| Hospital | Missed opportunities | Patient harm or death |
| GP Practice | Had toxic culture | Inquiry |
| Integrated care board | Ignored concerns | Senior resignation |
| etc | Mismanaged finances | Fine |
| Had operational failures | CQC inspection or censure | |
| etc | Workforce disengagement | |
| Postcode lottery | ||
| etc |
Familiar themes, so often repeated that we become immune to them, made explicit by the many enquiries into healthcare failures: Ely, Bristol Heart, Mid-Staffordshire, Morecambe Bay, Shipman. What have we leaned? More importantly, what have we done to prevent similar things happening again?
One of the themes of healthcare enquiries is an assessment of accountability: who knew about the failure and when? Or if the failure was unrecognised, who should have known and why didn’t they know?
Frequently after an enquiry there are resignations, retirements or dismissals of senior staff who are held accountable: the average NHS Trust Chief Executive is in post for about 3 years. Something has gone wrong so heads must roll. Accountability is clearly important – executives are defined by their ability to make significant organisational decisions and by inference therefore be responsible for them. It’s not unreasonable that executives and senior management are held accountable for failures that occur on their watch. The problem with this approach though is that an assessment of individual (or corporate) accountability is frequently insufficient to understand the causative factors in a failure. The system context in which decisions are made, strategies decided upon and priorities chosen is critical and many systematic factors are outside the direct control of even senior executives.
We have become familiar with the concept of a no-blame culture in healthcare even if it remains largely a unicorn concept: that people (staff, patients, the press, legal teams, politicians) might approach episodes of poor care or poor outcome with an open, curious and non-judgemental manner, searching for answers to make things better rather than focussing on liability. The benefits of this approach are well documented, and it is deeply culturally embedded in some industries, especially aviation, as the opening paragraphs of most air accident investigation reports clearly attest. By avoiding scapegoating we enable all colleagues to contribute to an investigation in a spirit of psychological safety, not worried about their career, their livelihood, even their liberty. In doing so we gain a wealth of system intelligence about reasoning, about the why and how, and not just the what.
In my experience most people try their best and while some are more capable than others, few professional people in healthcare make deliberately self-interested or reckless decisions whatever their seniority. Executives should listen and be curious about the impact of their decisions; they may need to be brave in their choices and carry them to a conclusion without being defensive. They need to be sure of their values and transparent in how these frame their decisions. But it’s not reasonable to expect them always to be right, and by extension not reasonable to blame them (unless they have been wilfully blind) for decisions that turn out to be wrong, even if they are accountable for them.
For a no-blame culture in enquiry to flourish, it requires an essential ingredient: trust. Without trust, enquiry becomes adversarial inquisition and opportunity for true learning is lost.
Decision makers must trust that their decisions, whether strategic, tactical or operational, will be reviewed objectively and without bias. Executive decisions are frequently made in the face of significant uncertainty, system volatility and outcome ambiguity so a decision that turns out to be hopelessly wrong (or have adverse unanticipated consequences) may still be made honestly and in good faith. In order to feel safe sharing information about how and why decisions were made, executives will need to trust the enquiry process, its chair and its scope and terms of reference.
Just as (if not more) importantly, the public need to trust that a process that does not result in blame and individual censure is not the same thing as a cover-up. They will need educating that in a complex system accountability is frequently delegated, diffuse and nebulous: a product of organic (and sometimes chaotic) organisational evolution rather than purposive design by an individual or group to whom responsibility can easily be apportioned. In the context of our current sociopolitical discourse, this is a hard sell.
How do we reconcile a no-blame culture (with all the system intelligence it brings) with the need for executive accountability? How high up an organisation should a no-blame culture extend? How can we maintain public faith in a process while enabling those experiencing it to speak freely and without anger, paranoia or fear. Only with trust.
I wonder if our failures to prevent recurrent harm in healthcare are related to our lack of trust, resulting in a willingness to seek accountability and then apportion blame. Blame allows us to embody the failings of a system in an individual. It gives the system a face and a focal point for our distress, anger or confusion. But the risk is that having fulfilled our atavistic desire for redress we lose interest in the hard work of system redesign, cultural change and investment in people, process and capital that might actually make a difference. Removing accountable staff is simple, easy, and cheap. System change is often complex, challenging and expensive, possibly prohibitively so.
So are we ready for a no-blame culture? Are our politicians, our profession, our legal system and the public really aware of the revolution in mindset needed? Do we have the trust in institutions, experts and process that such a culture requires? I am not hopeful.
Despite the exhortations from the great and the good, from multiple Secretaries of State for Health, the reports from august bodies, the hand wringing and introspection, we continue to blame and we continue to fail. Is it inevitable that, just as with politicians, the careers of all senior healthcare executives end in failure? Much more importantly, is it inevitable that we will keep not learning the same lessons over and over again?
Without trust, I fear it is.